| Preparation |
Just what you need to know ! |
Pressure is defined as the force per unit area.
Pressure = Force / Area
The SI unit of pressure is Pascal (abbreviated as Pa) and is named after the scientist Blaise Pascal.
One Pascal is defined as one Newton per square meter. So, 1 Pa = 1 N m-2.
Another unit of pressure that is used is bar. Note that 1 bar = 105 Pa and 1 bar = 1000 millibars.
Pressure is isotropic, which means that it acts equally in all directions.
Thus, liquids exert the same pressure in all directions at a given depth.
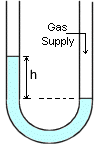 Manometer is the instrument for measuring pressure in terms of the difference in fluid levels between the vertical arms of a glass U-tube. Manometer is the instrument for measuring pressure in terms of the difference in fluid levels between the vertical arms of a glass U-tube.
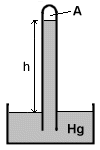 Barometer is the instrument used to measure atmospheric pressure. Barometer is the instrument used to measure atmospheric pressure.
Atmosphere pressure is the force exerted by the atmosphere per unit area on the surface of the earth.
Atmospheric pressure is about 105 Pa at sea level, and decreases with increasing altitude.
The average atmospheric pressure at sea level corresponds to 760 mm Hg (which is the vertical height of mercury in a barometer) or 10.336 m of vertical height of a water column.
To correctly measure the atmospheric pressure, the space (labeled A in the figure) above the mercury level in a barometer is essentially vacuum.
Otherwise, the fluid in the space will exert a pressure on the mercury surface.
Barometers modified to measure the altitude (or the height above sea level) are called altimeters.
An aneroid barometer measures air pressure by its action on the lid of a thin metal box evacuated of air (containing vacuum). Aneroid means 'not employing fluid'.
Sphygmomanometer is the apparatus used to measure blood pressure.
Fluid pressure on a surface is due to the force acting on a unit area by the molecules of the fluid (liquid or gas) continuously hitting the surface.
Pressure at a point in a fluid is directly proportional to the density of the fluid and the depth of the point. Mathematically,
P = ρ g h, where
P is the fluid pressure at a point,
ρ is the density of the fluid,
g is the acceleration due to gravity, and
h is the depth of the point.
In a closed vessel, the volume of a gas decreases with increasing pressure and vice-versa.
Boyle's law states that the volume of a fixed mass of an ideal gas is inversely proportional to the pressure, provided the temperature is held constant. Mathematically,
PV = constant (at a fixed temperature), where
P and V are the pressure and volume of the fixed mass of ideal gas, respectively.
Physics Quiz on Pressure. |

